In the field of allyl-thiol click on chemical post-modification ir, click chemistry has emerged as a powerful and efficient method for joining two molecular entities with high selectivity. One of the widely explored variations of click allyl-thiol click on chemical post-modification ir click reactions, where an allyl group and a thiol functional group undergo a specific reaction to form a new bond. This reaction type is highly valuable in post-modification of various chemical compounds, materials, and polymers, especially when designing complex molecular architectures.
In recent years, there has been growing interest in applying allyl-thiol click on chemical post-modification ir spectroscopy studies, particularly for understanding how these reactions can alter material properties, structure, and functionalization. This article delves into the details of allyl-thiol click chemistry, its utility in chemical post-modification, and the role of IR spectroscopy in monitoring and analyzing these reactions.
Fundamentals of allyl-thiol click on chemical post-modification ir
What is Allyl-Thiol Click Chemistry?
Allyl-thiol click on chemical post-modification ir involves the reaction between an allyl group (–CH2CH=CH2) and a thiol group (–SH) to form a thioether bond (R–S–R’). This reaction is particularly attractive because it proceeds under mild conditions, without the need for high temperatures or the presence of toxic catalysts. It offers high regioselectivity, ensuring that the desired product is formed without significant byproducts.
The core concept behind click chemistry, including the allyl-thiol click on chemical post-modification ir, is the idea of making reactions fast, efficient, and orthogonal to other chemical functionalities, allowing scientists to perform precise chemical modifications without affecting the rest of the molecule.
Mechanism of the Reaction
Allyl-thiol click reactions typically proceed via a radical mechanism. The thiol group, which acts as a nucleophile, attacks the double bond in the allyl-thiol click on chemical post-modification ir group, leading to the formation of a new sulfur-carbon bond. Depending on the reaction conditions, a radical initiator like azobisisobutyronitrile (AIBN) may be used to facilitate this process. Other variations include light or heat-induced reactions that can initiate the radical formation required for the click reaction to occur.
Advantages of allyl-thiol click on chemical post-modification ir
- High Efficiency: The reaction proceeds rapidly, leading to high yields without extensive purification steps.
- Mild Conditions: The ability to perform these reactions under ambient conditions without harsh reagents is particularly appealing for sensitive substrates.
- Orthogonality: allyl-thiol click on chemical post-modification ir reactions are orthogonal to many other chemical reactions, making them ideal for post-functionalization processes.
- Versatility: This reaction can be used to functionalize a variety of molecules, including small organic compounds, polymers, proteins, and surfaces.
Chemical Post-Modification via allyl-thiol click on chemical post-modification ir
What is Chemical Post-Modification?
Chemical post-modification refers to the alteration of pre-synthesized compounds or materials through additional chemical reactions. These modifications are performed after the initial synthesis to introduce new functional groups, tune physical properties, or enhance the material’s performance. allyl-thiol click on chemical post-modification ir reactions are particularly useful for post-modification since they allow for selective and precise functionalization of complex molecules or surfaces.
Applications in Polymer Chemistry
One of the most prominent areas where allyl-thiol click chemistry has found success is in polymer chemistry. Post-modification of polymers allows for the fine-tuning of physical and chemical properties, such as solubility, thermal stability, and conductivity. By introducing thiol groups onto a polymer backbone and then using allyl-thiol click on chemical post-modification ir, it is possible to graft various functional groups onto the polymer to create materials with unique properties.
For instance, this approach has been employed to modify hydrogels, making them responsive to environmental stimuli like pH or temperature changes. In addition, polymers used in drug delivery systems can be modified to achieve controlled release of therapeutic agents, enhancing their efficiency and reducing side effects.
Surface Functionalization
In material science, surface functionalization is crucial for creating interfaces with specific properties, such as hydrophilicity, hydrophobicity, or bioactivity. allyl-thiol click on chemical post-modification ir has been used to modify surfaces with high precision. This approach has been particularly beneficial for developing sensors, where the surface needs to be tailored to detect specific molecules or changes in the environment.
For example, gold nanoparticles or silicon surfaces can be functionalized with allyl-thiol click on chemical post-modification ir groups, and subsequent reaction with thiols allows for the attachment of biomolecules or other functional units. This technique has been widely applied in creating biosensors for medical diagnostics and environmental monitoring.
Monitoring Allyl-Thiol Click Reactions with Infrared Spectroscopy
Importance of IR Spectroscopy
Infrared (IR) spectroscopy is one of the most powerful tools for characterizing chemical reactions and post-modifications. It measures the absorption of IR radiation by the chemical bonds in a molecule, providing a molecular fingerprint that can be used to identify functional groups and monitor changes in chemical structure.
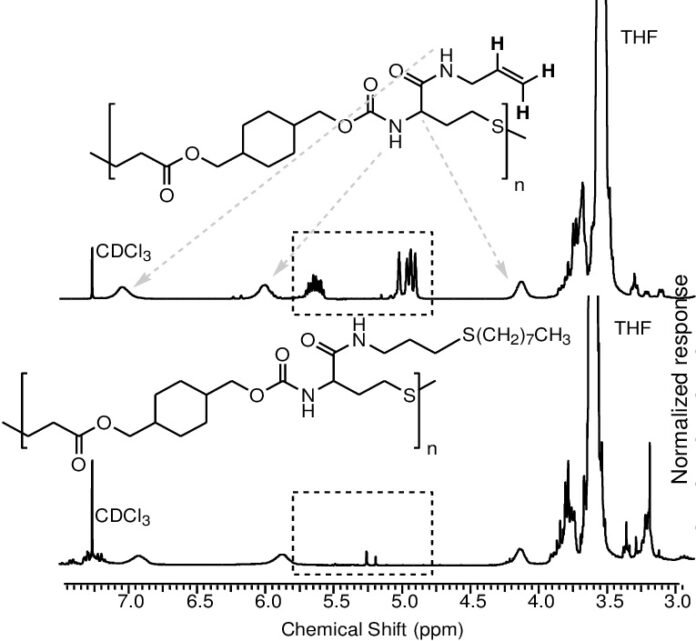
In the context of allyl-thiol click chemistry, IR spectroscopy plays a vital role in monitoring the progress of the reaction and confirming the success of the post-modification. The disappearance of specific bands associated with the thiol group or the allyl group, coupled with the appearance of new bands corresponding to the newly formed thioether bond, provides clear evidence of the reaction.
Key IR Bands for Allyl-Thiol Click Chemistry
The following IR bands are of particular interest when monitoring allyl-thiol click reactions:
- Thiol (–SH) Stretch: The thiol group typically exhibits a characteristic stretch around 2550–2600 cm⁻¹. As the thiol reacts with the allyl group, this band decreases in intensity or disappears altogether.
- Allyl Group (C=C): The double bond in the allyl group gives rise to a signal between 1640–1680 cm⁻¹. Monitoring this band helps track the consumption of the allyl group as the reaction proceeds.
- Thioether (R–S–R’): After the formation of the thioether bond, new bands appear in the region around 700–800 cm⁻¹, confirming the presence of the sulfur-carbon bond.
By comparing the IR spectra before and after the reaction, researchers can confirm whether the allyl-thiol click reaction has taken place and to what extent the post-modification was successful.
Time-Resolved IR Spectroscopy
In some cases, time-resolved IR spectroscopy can be used to monitor the reaction kinetics in real-time. By taking IR measurements at regular intervals during the reaction, it is possible to observe the gradual disappearance of reactant bands and the concurrent appearance of product bands. This approach provides valuable insights into the rate of the reaction and can help optimize reaction conditions.
Challenges and Considerations in Allyl-Thiol Click Reactions
Selectivity and Side Reactions
While allyl-thiol click chemistry is highly selective, certain conditions may lead to side reactions or incomplete conversions. For instance, the presence of oxygen can interfere with the radical mechanism, leading to unwanted oxidation of the thiol group. Careful control of the reaction environment, such as performing the reaction under inert atmospheres (e.g., nitrogen or argon), is essential to prevent such issues.
Compatibility with Different Functional Groups
The success of the post-modification depends on the compatibility of allyl-thiol click reactions with other functional groups present in the molecule or material. While the orthogonality of click chemistry generally allows for selective modifications, some functional groups, such as amines or carboxylic acids, may need to be protected during the reaction to prevent unwanted side reactions.
Scalability of the Reaction
For industrial applications, scalability is an important consideration. Allyl-thiol click reactions are generally well-suited for scaling up due to their mild reaction conditions and high efficiency. However, the choice of solvent, initiators, and reaction setup must be carefully optimized to ensure that the reaction remains efficient and cost-effective at larger scales.
Applications of Allyl-Thiol Click Chemistry in Various Fields
Drug Delivery Systems
Allyl-thiol click chemistry has been extensively used in the development of drug delivery systems, where post-modification of polymers or nanoparticles is required to improve drug loading, release, and targeting capabilities. Functionalization of the surface of drug carriers with targeting ligands through allyl-thiol click reactions has shown promise in enhancing the specificity of drug delivery to cancer cells.
Bioconjugation
In biochemistry, allyl-thiol click chemistry has been employed for the site-specific conjugation of proteins, antibodies, and other biomolecules. By attaching functional groups such as fluorescent labels or drugs to a biomolecule, researchers can study cellular processes, track the localization of molecules in biological systems, or develop new therapeutics.
Material Science
The ability to modify surfaces and polymers through allyl-thiol click reactions has made this chemistry an important tool in material science. From self-healing materials to responsive coatings and sensors, the versatility of allyl-thiol click reactions enables the creation of novel materials with tailored properties for specific applications.
Conclusion
Allyl-thiol click on chemical post-modification ir offers a versatile and efficient method for chemical post-modification, particularly in the fields of polymer chemistry, surface functionalization, and bioconjugation. The mild conditions and high selectivity of this reaction make it ideal for modifying complex molecules and materials. IR spectroscopy, as a monitoring tool, plays a crucial role in ensuring the success of these modifications by providing real-time information on reaction progress and product formation.
The applications of allyl-thiol click on chemical post-modification ir are vast, ranging from drug delivery systems to advanced material design. As research continues, this technique will likely see even broader use in both academic and industrial settings, offering new possibilities for creating tailored materials and functional systems.